Fusion Antibodies: Quality By Design
This article featured in the September 2022 edition of Nature Biopharma Deal.
From antigen design to cell-line development for the successful production of biologics.
Selecting a therapeutic antibody that will become commercially successful is often a huge gamble, with candidates potentially failing at any stage of development. Those that are rushed through the preclinical stages, in particular, may have biophysical properties that turn out to hinder downstream manufacture or cause unwanted immunogenicity. Problems with the primary sequence of a protein, for example, can cause issues such as isomerization and glycosylation, while design weaknesses in the tertiary structure can result in instability that leads to aggregation—any of which could hamper later development, clinical-trial outcomes, or the manufacture of a therapeutic candidate.
With safety, stability, functionality and scalability crucial for commercial success, neglecting to fully consider these at the preclinical stage is a false economy. Indeed, hundreds of millions of potentially wasted dollars could be saved if companies took a more holistic approach to early-stage discovery/development by fully considering all the obstacles ahead.
“Progressing antibodies to the clinic is challenging, but we have a profound understanding of the hurdles and how to address them to unlock the path to the clinic with a potential blockbuster,” said Hugh Morgan, Director of Operations.
Putting function first
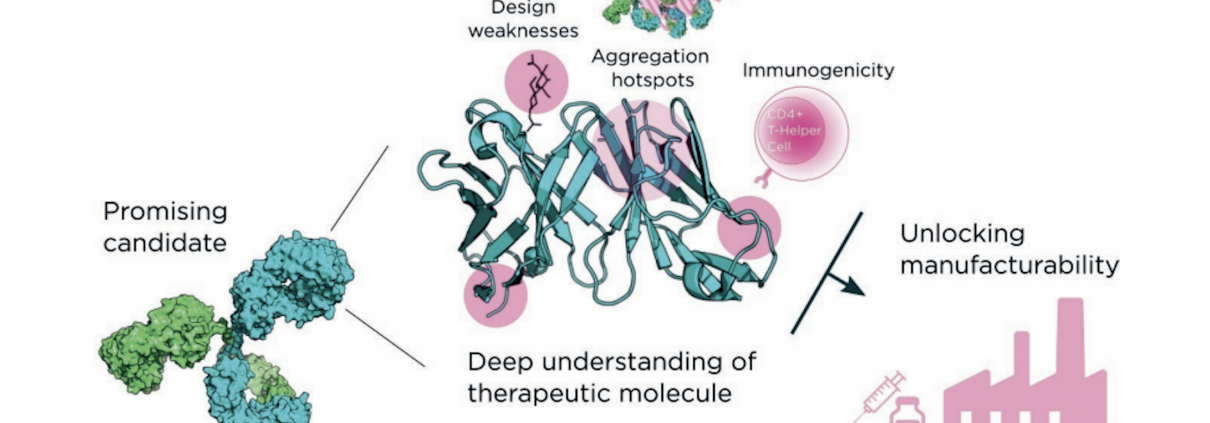
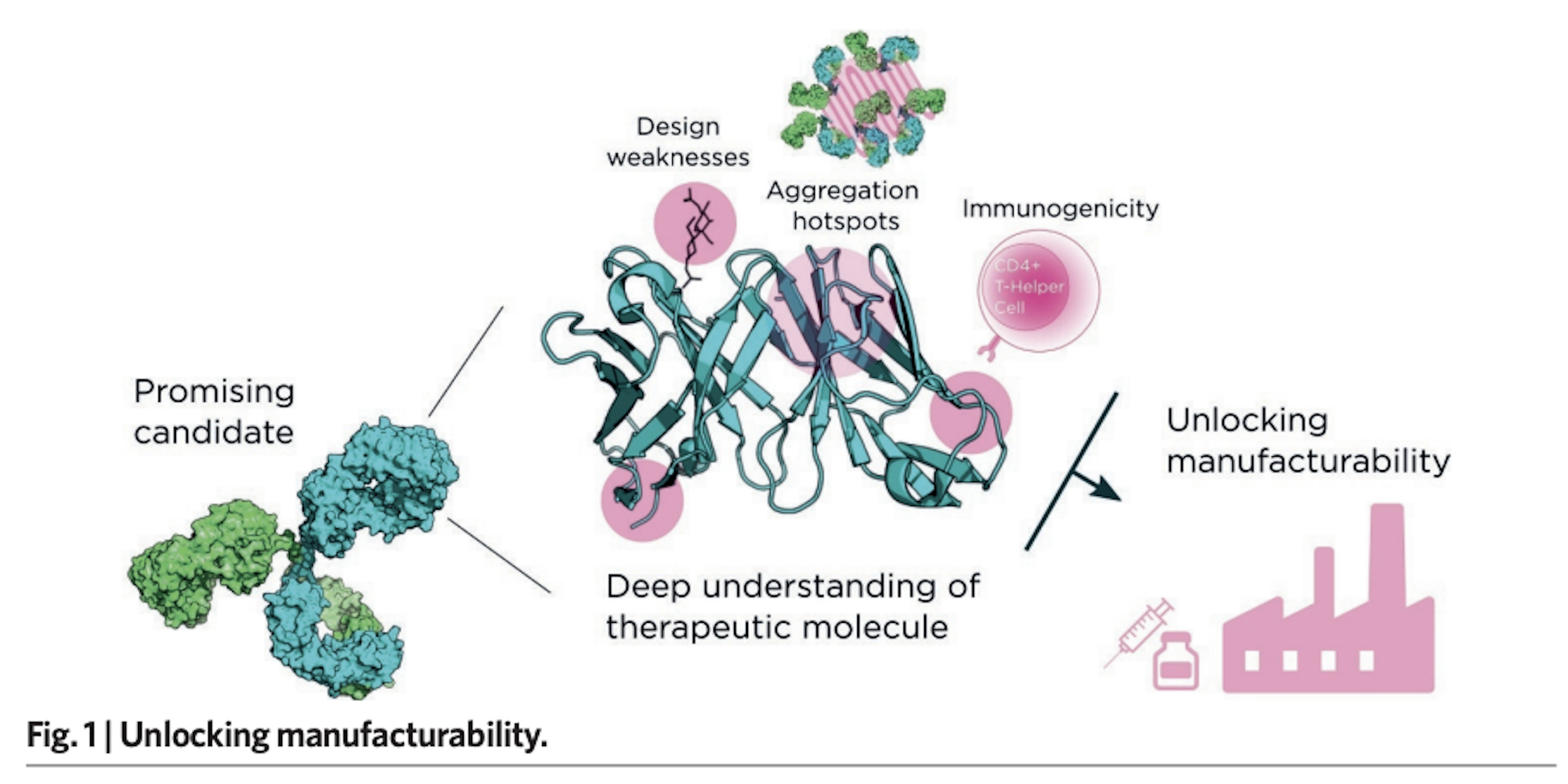
Having successfully taken hundreds of projects through the critical early discovery stages, and drawing on an array of innovative services and proprietary platforms, Fusion Antibodies is well placed to provide bespoke and responsive guidance on the entire preclinical pathway, from antigen design to cell-line development (Fig. 1).
As an R&D-partnering organization with extensive expertise in antibody discovery and engineering, Fusion Antibodies’ approach and understanding of different antibody attributes maximizes the safety, efficacy and manufacturability of biologic drugs. Understanding these issues as early as possible in the drug discovery and development process is vital to later success. With in-depth knowledge of the overarching clinical development obstacles facing drug developers, Fusion Antibodies designs and produces therapeutic molecules with the properties required for their successful development, manufacture and function, thus avoiding unforeseen issues downstream.
The company’s platforms, for example, have built-in sequence liability screens to ensure they generate non-immunogenic molecules with the best possible sequences. And, to avoid structural liabilities, Fusion Antibodies utilizes state-of-the-art three-dimensional (3D) protein structural modelling tools, rather than relying on guesswork for epitope prediction. It also uses mature human frameworks that have undergone the natural interrogation of evolution (rather than a germline approach) to produce stable, developable molecules.
“It is critical to mitigate the risks and get these foundations right,” said Morgan. “Investing in developing an ideal drug molecule at this stage will save both time and wasted expenditure later and significantly increase chances of success.”
Figure 1
Unique technology platforms
Also among Fusion Antibodies’ suite of capabilities is its unique approach to affinity maturation. Instead of phage display, its rational affinity maturation platform, RAMP, harnesses the power of the natural somatic hypermutation space, rapidly introducing diversity into not only the complementarity determining regions (CDRs) but also the framework regions—an approach not found elsewhere.
With respect to humanization, Fusion Antibodies utilizes an in-silico library of mature human frameworks to deliver diverse humanized variants that retain function and maintain an affinity within twofold, regardless of parental species.
The company is also developing a new antibody library in a powerful mammalian cell display platform. Therapeutic candidates raised from the library will be in a commercially relevant immunoglobulin G (IgG) format, contain important post-translational modifications required for function, and be directly translatable to the final manufactured product at the earliest stages of drug development. The platform will open up molecules that would be impossible to discover in hybridoma or phage-display platforms, allowing a wider selection of discovery options and ultimately maximizing the chances of therapeutic success.
Going forward
Developability is especially important when it comes to difficult-to-engineer molecules, such as fragments, fusion proteins and multi-specifics. These are likely to dominate the future antibody therapeutic market, but many of them express poorly and have aggregation issues leading to delays to projects.
Fusion Antibodies utilizes unique talent in understanding antibody structure, and experience in molecular modelling, to design and produce advanced bi- and multi-specific molecules. By adapting the design process to specific needs, the company can generate complex molecular formats with vast varieties of different specificities, valencies and structures, the design of which can be exquisitely tailored to the intended therapeutic use. Additionally, Fusion Antibodies has harnessed a sophisticated protein-engineering approach to standardize antigen expression based on its robust engineering technology underpinning bi-specific antibody design. The team has developed a knobs-into-holes (KIH) engineering platform for difficult-to-express antigens, whilst linking a reporter arm to streamline later steps in the discovery process. The modular design allows for the generation of a toolkit of molecular formats with specific reporter modules, enabling a highly tailored approach to the discovery process. This approach will play an integral role in streamlining Fusion Antibodies’ future drug development pathway.
To summarize, Morgan said “We produce molecules that work! They are immunogenically de-risked and manufacturable—considering attributes that are often missed by others—and offer you the best chance of clinical and commercial success.”
Contact Chris Winch, Commercial Director of Fusion Antibodies to find out more.