Stepping outside of the CDR to create naturalised libraries and humanized antibodies
With their high specificity, efficacy and safety profiles, it’s no wonder that antibodies have become the biggest selling drugs in recent years. Advances in antibody discovery, selection and manufacturing have catapulted therapeutic antibodies to become the primary treatment for several diseases and the market shows no sign of abating, with the industry expected to be worth $300 billion by 2025.
Mammalian antibody libraries are critical tools in the development of novel antibody therapies. By providing the platform for discovery and selection, these libraries help streamline and expedite the identification and pre-clinical optimisation of humanized antibodies. However, with limited numbers of available mammalian cell lines, it is difficult to achieve high diversity and maintain transfection efficiency.
Traditional libraries target complementary determining regions (CDRs) for mutational variation which limits potential diversity. What’s more, although CDRs are important for antigen binding, true specificity is much more complicated. Next-generation, synthetic mammalian libraries are taking a new approach to increase the specificity and affinity of manufactured antibodies. By increasing somatic hypermutation to mimic the natural mutation repertoire, large libraries of complete IgG, fully-human antibodies are being generated with high affinity and low immunogenicity.
Traditional techniques are limited by fragmented approaches
Traditional synthetic antibody libraries create diversity by concentrating mutations within the CDRs. Once suitable human germline frameworks are selected, oligo-DNA cassettes are created for the CDRs. Diversity is then introduced through the use of NNK/NNS degenerate codons or error-prone polymerase chain reaction (PCR) techniques that create random amino acid mutations. Although some libraries now have biases towards the creation of certain amino acids within the CDR, the diversity is still only limited to these areas and the stark contrast in diversity between the framework and CDR regions is clear.
While CDRs are undoubtedly important for antigen-binding, framework areas contribute greatly to binding affinity and, using traditional techniques, these areas are largely ignored (see Figure 1). In the IGHV3-23 version antibody, widely used in therapeutics, mutations are almost solely seen in the CDR1, 2 and 3 regions of the variable heavy domain with little to no mutation seen in the framework areas.
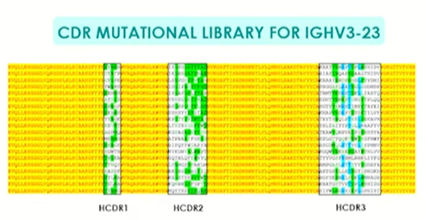
Figure 1: Typical mutational pattern in a traditional synthetic mammalian library version of heavy gene IGHV3. Mutations are indicated by the white, green and blue colours and diversity is found only with the CDR regions.
Traditional display platforms are often limited to scFv or Fab fragments rather than full immunoglobulin (IgG) constructs. These fragments need to be converted to full IgG before they can be used as a marketable therapy, a process which is not always straightforward. To create antibodies that fully mimic the effectiveness of the B-lymphocyte’s response to antigens, we must turn to nature and generate libraries and techniques that mirror this response more closely.
Mammalian libraries that mimic natural mutations
Next-generation mammalian libraries are now being designed to align with natural repertoires, with mutations created throughout the heavy chain and, in particular, somatic hypermutations in the framework areas. In patient responses to SARS-CoV-2 spike protein, IGHV3-23 antibodies show mutations throughout the CDR and framework, with significant amounts of mutation in CDR 3 (figure 2). Successful phage displays, using libraries from COVID-19 patients, have already resulted in the creation of neutralising antibodies with promising results of treatment and immunising potential. These experiments demonstrate the inherent diversity needed for effective naïve human library design and their resulting antibody treatments.
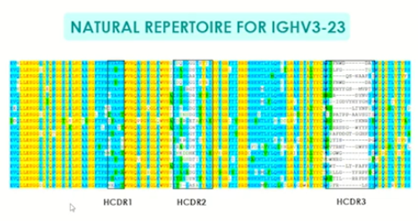
Figure 2 Natural repertoire of IGHV3-23 mutations – demonstrating significant mutations throughout the CDRs and framework regions.
Fusion Antibodies has developed an optimised mammalian antibody library yielding complete, fully human antibodies. The library is designed for market optimisation and has been formed by selecting the most commonly used antibodies with the greatest market and downstream manufacturing potential. As well as choosing readily marketable antibodies, heavy chain CDR3 amino acid lengths were selected to mimic the natural response seen in humans. Guided by affinity maturation and humanisation platforms, mutational variation was added to the framework regions, along with the addition of separate CDR cassettes to mimic the natural genetic repertoire. The resulting antibodies show high affinity with low immunogenicity and are free of sequence liabilities.
COVID-19 treatments are just the beginning
COVID-19 neutralising antibodies are just tip of the treatment potential offered by fully humanized antibodies, created through this next generation of naïve mammalian libraries.
By screening antibody targets against whole, human antibodies the number of steps needed to discover new antibodies is greatly reduced, eliminating the need for platform switching and the reformatting required by some other approaches. By optimising future marketability at the design phase, antibodies are selected based on their affinity, downstream processing compatibility and market potential.
Next-generation mammalian libraries that maximise diversity, both in the CDRs and through somatic hypermutation in the framework areas, have the potential to increase the effectiveness of antibody treatments. With faster development timeframes and naïve libraries that mimic natural antibody repertoires, we can look forward to a future of treatments and diagnostics with higher affinity, selectivity and stability.