Bispecific antibodies are engineered to combine two epitope targeting regions into the same molecule and have long held out promise of expanding the potential of conventional monoclonal antibody therapeutics. Intelligent engineering of these molecules can go even further with the design of molecules with several epitope targeting regions termed multispecifics. In fact, the number of specificities, valency, and structure of these multispecifics can be varied in such a way as to allow an extensive panoply of potential molecular formats, the design of which can be exquisitely bespoke to the intended therapeutic use.
Uses of bi-/multispecifics
The industry pipeline for multispecifics has matured to a point in which many molecules from different multispecific platforms are poised to deliver new therapeutic products. The industry appetite for such molecules is indicative of the predicted therapeutic value of multispecifics, with the promise to propel the field, especially in oncology, to better clinical outcomes. Courtesy of their ability in binding multiple antigens at the same time, this diverse family of molecules can act in a variety of mechanisms by manipulating the spatial and temporal resolution of target molecules and cells.In this way, multispecific antibodies can bridge gaps or act as circuit breaks in signaling cascades, bring receptor molecules together, form multiple blocks on disease-related pathways, coordinate the interface between different cell types, to illustrate a limited few.More specifically, multispecific antibodies have huge promise as cancer therapeutics. Mechanisms of action include selecting for tumor cells via multiple targets to increase specificity and potentially perturb refractory or resistant forms of cancer, bringing together tumour cells and T-cells and/or other effector cells such as NK cells to coordinate multiple complimentary immune mechanisms, the presence of several specificities also allow the increased acuity of targeting a tumour cell alongside the ability to target the tumour microenvironment and limit off-target toxicity.
Difficulties engineering bispecifics
Despite the promise of multispecific antibodies, few have been approved at the present time. As of Q3 2021, only 4 bispecific molecules have been approved in the EU or US with 2 more in regulatory review1. Of these, the blockbuster HEMLIBRA®(emicizumab) for heamophilia has sales of >$500m a year2, illustrating the huge potential value of such molecules. The historical scarcity of multispecifics progressing to regulatory approval is in large part due to the considerable difficulties in producing such highly non-native molecules. The engineering and production process of bi-and multispecific molecules traditionally produces lower yield and purity products, this is mainly due to the problem of incorrect chain assembly plus additional aggregation and stability issues limiting the manufacturability of such therapeutics. However, with recent intelligent engineering advances, there are now several clinically validated multispecific platforms that circumvent some of the issues described. In fact, presently there are over 100 bispecific antibodies in the clinical pipeline ranging from tandem single-chain variable fragments (scFv) to full-length immunoglobulins with dual variable domains. Such molecules are also on an increasing trajectory. As of 2018, bispecific molecules accounted for 25% of the total antibody therapeutics in development, up 150% from the early 2010s1. Many of these therapeutics are poised to gain approval within the next decade as current generation bispecifics have almost identical rates of progress though clinical trials as other monoclonal antibody therapeutics.
Overcoming engineering difficulties at Fusion
At Fusion Antibodies, we have extensive expertise and experience in the use of many established multispecific technologies such as Knobs in Holes platforms (KIH) but can also utilise novel, non-propriety design strategies dependent on a client’s requirements. With a quality by design approach, we employ our in silico and protein engineering expertise to design and optimise an engineering program ideal for an antibody candidate, shaped with the endpoint in mind. This quality first approach leads all the way through to protein production in our transient gene expression (TGE) services where we offer optimisation of bespoke expression and purification strategies, of huge value for such challenging molecules. The complete process of antibody engineering is devised with the end in mind with considerations about scalability and manufacturability. The ultimate aim of these approaches is rooted in increasing the chances of therapeutic success.
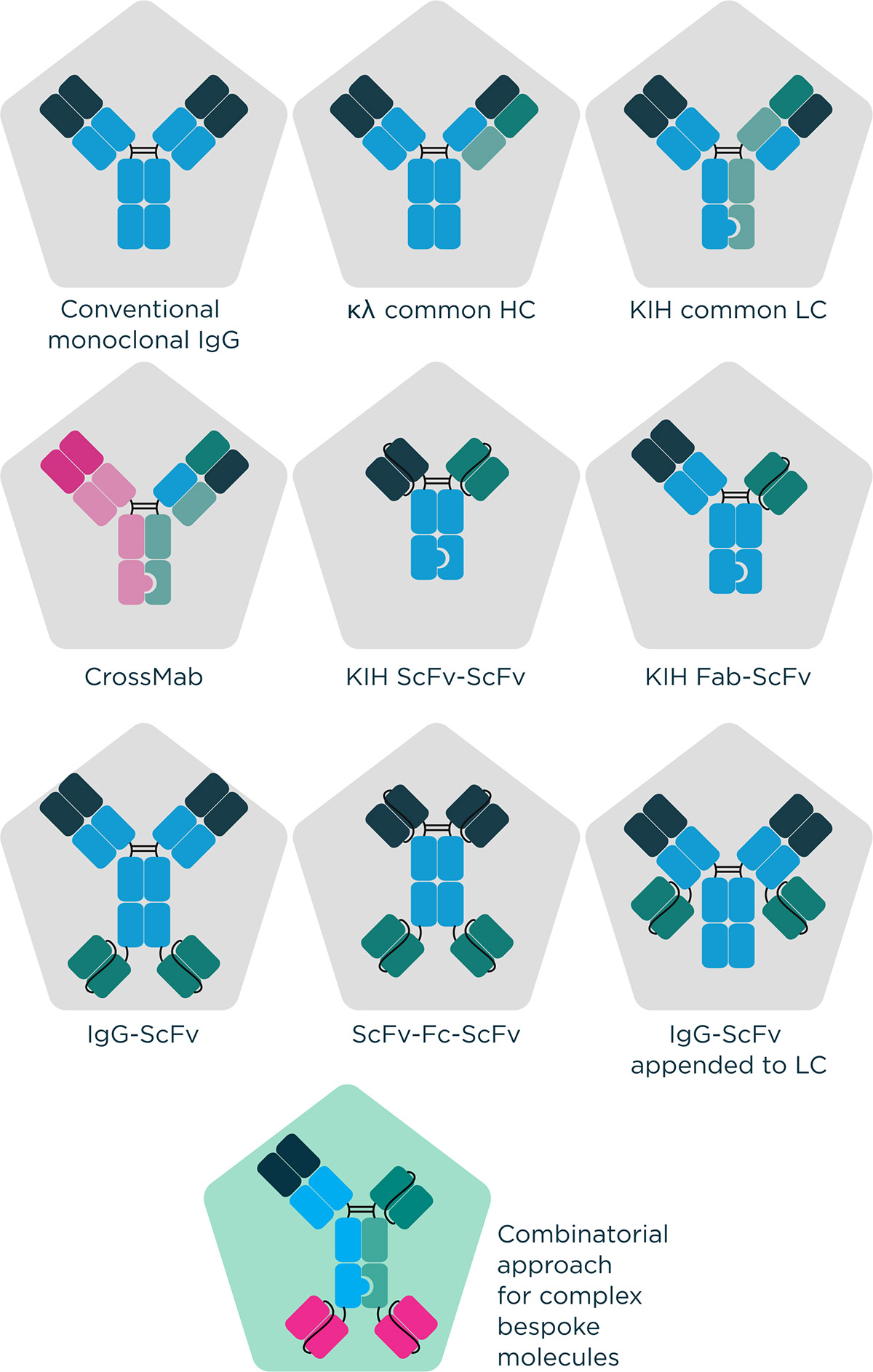
Figure 1 – Examples of molecular formats that can be engineered by Fusion Antibodies.
Email info@fusionantibodies.com today to learn how we can help with your multispecific antibody development program.
-
https://www.antibodysociety.org
-
https://www.roche.com
