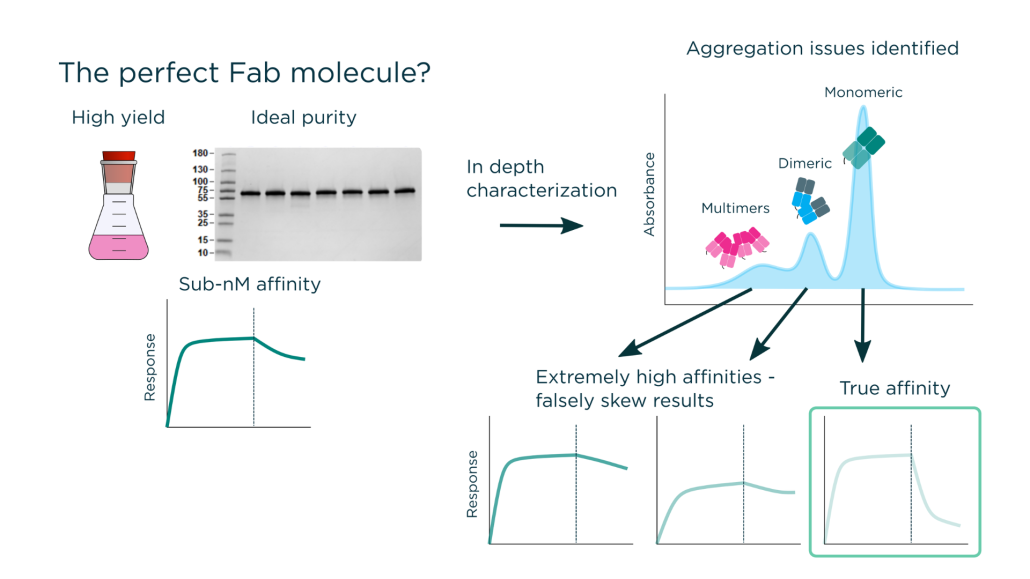
 https://fusionantibodies.com/wp-content/uploads/sequencing-scaled-1.jpeg
1707
2560
mint_design
/wp-content/uploads/2022/01/fusion-antibodies-logo.svg
mint_design2021-07-14 11:52:322023-05-02 15:26:04Stepping outside of the CDR to create naturalised libraries and humanized antibodies
https://fusionantibodies.com/wp-content/uploads/sequencing-scaled-1.jpeg
1707
2560
mint_design
/wp-content/uploads/2022/01/fusion-antibodies-logo.svg
mint_design2021-07-14 11:52:322023-05-02 15:26:04Stepping outside of the CDR to create naturalised libraries and humanized antibodiesOptimize your antibody to be the best
Find out more about partnering with Fusion Antibodies or contact us to start a project
JOURNEY WITH US
CONTACT US
info@fusionantibodies.com
+44 (0)28 9043 2800
Investor and Media
+44 (0)20 7933 8780
fusion@walbrookpr.com
Fusion Antibodies PLC, Springbank Industrial Estate, 1 Springbank Rd, Dunmurry, Belfast BT17 0QL